Hérouville-Saint-Clair
The Hérouville-Saint-Clair site, located near Caen, in Normandy, specializes in the manufacture and filling of injectable and non-injectable sterile liquid products. It has a pharmaceutical surface area of 17,900 m² over 10 hectares and employs 290 people*.
This additional site acquired in 2017 from MSD, allowed Cenexi to significantly increase its syringe, vial, and ampoule filling capacity. This site also manufactures creams, ointments, lotions, drops, and syrups to treat allergies, inflammatory and infectious diseases, and cancers.
The site has increased its capacity in sterile products in all forms, in particular, by introducing the manufacture of an ophthalmic gel.
In December 2021, Hérouville reached a major milestone in its development by obtaining FDA accreditation for the production of a sterile injectable product, aseptically packaged in vials for the USA. It had already obtained accreditations from the Brazilian, French, and Russian health authorities.
Finally, the ANSM recently authorized the site to manufacture and certify biological products (immunological and biotechnology).
The physicochemistry and microbiology control laboratories also allow on-site analyzes (packaging materials, raw materials, semi-finished products, and finished products).
* Figures as of January 1, 2023.
Key figures*
million units produced every year
destination countries, including France, the main beneficiary
employees
Pharmaceutical forms
- Ampoules
- Vials
- Pre-filled syringes
- Creams
- Ointments
- Syrups
- Lotions
- Drops
- Gels
Most recent investments
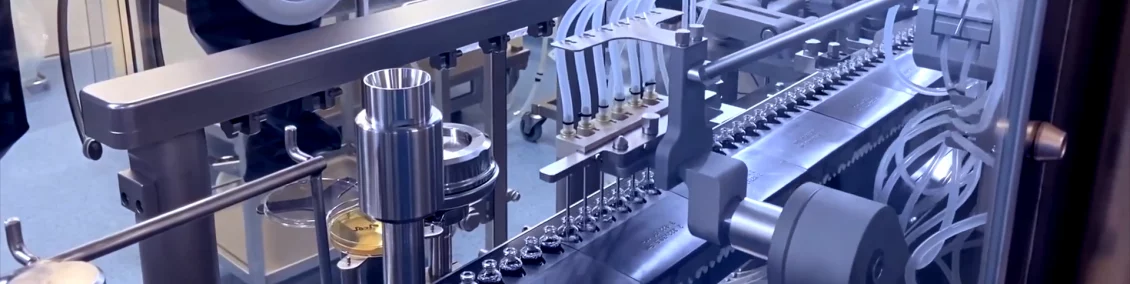
- A new vial line, with an available filling capacity of 40 million 2 ml bottles per year, entered into service in late 2019 to better position the site in its market. The site’s first biological products, monoclonal antibodies, will be filled on this line
- A new ampoule line entered into service in 2021, in response to the increase in the activity of existing customers
- The sterile block was extended for the manufacture and filling of gel tubes, with the purchase of filling equipment
Hérouville-Saint-Clair and the treatments against COVID-19
The clinical batches of CoVepiT will also be manufactured in Hérouville, on a production line with an available filling capacity of 40 million vials (2 ml) per year. This 100% French next-generation multi-target, multi-variant vaccine, intended to cover all existing and future variants of SARS-CoV-2, is being developed by OSE Immunotherapeutics, a Nantes start-up specializing in novel immunotherapies in the clinical phase. According to this agreement entered into in July 2021, Cenexi will implement the peptides and their sterile emulsion and fill the clinical batches of CoVepiT used in the phase 1 clinical trial, and potentially in the subsequent clinical phases subject to positive results in this first phase.
> Learn more about the partnership with OSE Immunotherapeutics.
Finally, it is in Hérouville that one of the most promising treatments against severe forms of Covid-19 will be manufactured for the next five years, following a partnership entered into in February 2022 between Cenexi and Humanigen, a US clinical-stage biopharmaceutical company specializing in the prevention and treatment of overactive immune responses. This agreement concerns the transfer of technology and expertise from Humanigen to Cenexi in order to manufacture the batches on site. Cenexi could also handle other steps, such as regulatory services, or even, in the long term, expand its services in order to manage the entire supply chain of this drug in France and Europe.